We are intrigued by ribosomes, the cell's protein factories, which are the most abundant machinery in the cytoplasm, as well as the nucleolus, the largest hub inside the cell's nucleus. Our objective is to understand how they interact with other cellular components and influence organism growth, energy processes, and chromatin structure. To achieve this, we utilize techniques from genetics, genomics, microscopy, and computer science.
1- A Ribosome Synthesis Checkpoint: Impacts on Post-Embryonic Growth
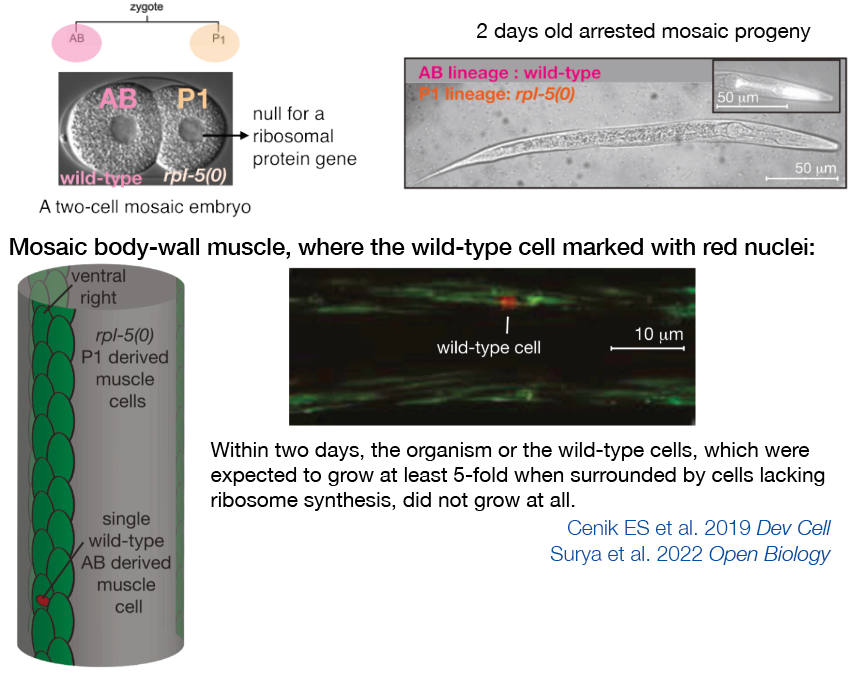
When we generate mosaic embryos with cells that are both capable and incapable of new ribosome synthesis, these embryos develop up to an early larval stage, drawing upon the deposition of maternal ribosomes. However, their postembryonic growth is stunted. Within two days, even the wild-type cells, which, under typical conditions, would grow at least 5-fold when surrounded by cells that lack ribosome synthesis, exhibit no growth. This suggests that if certain cells within the organism don't produce ribosomes, the growth of the entire organism is halted. We term this phenomenon the "ribosome synthesis checkpoint," distinct from the insulin pathway-mediated nutrition checkpoint. This checkpoint serves as a control mechanism, ensuring that ribosomes, essential for protein synthesis and cellular function, are produced as and when needed throughout the organism.
Questions
How do organisms inhibit ribosome activity in regular cells?
What controls the checkpoint that is independent of nutrition?
What triggers the signals and forces transmitted between cells?
A Key Role for Epidermal Secretion: Regulating Protein Translation and Inducing Reversible Quiescence in the Organism
Controlling When and Where Ribosomes are Made
When ribosome synthesis is intentionally suppressed in tissues of equal volume, body proportions remain consistent, suggesting a coordinated regulation of body ratios. Additionally, the epidermis alone can initiate a reversible quiescent response that is independent of the organism's nutritional status. In this state, the worms can survive and recover for up to five days.
Inhibiting ribosome synthesis in the epidermis halts protein synthesis and growth throughout the organism
When new ribosome synthesis is specifically inhibited in the epidermis, the protein synthesis across the entire organism is significantly reduced, leading to a substantially stunted growth, which is reversible. Interestingly, during epidermis-specific ribosome synthesis inhibition, many secreted proteins and dense core vesicle components show increased abundance at the protein level. Furthermore, the reversible growth checkpoint can be partially reversed when the secretion of dense core vesicles or a secreted protein called retinoic acid binding protein (FAR-1) is reduced specifically in the epidermis.
Questions
What causes ribosomes to remain inactive during quiescence?
How is the dense core vesicle pathway activated in response to ribosome synthesis inhibition?
2- Interplay Between Nucleolus and Chromatin
Does the nucleolus, the nucleus's central hub, actively contribute to organizing genetic material?
We've built a user-friendly analysis tool, SRAtac, an end-to-end customizable analysis pipeline for alignment and ATAC-seq analysis across different model organisms. You can find the SRAtac tool, at the following GitHub repositories: https://github.com/trev-f/SRAlign, https://github.com/trev-f/SRAtac
We used SRAtac to investigate the effects of nucleolar modifications on chromatin accessibility. Interestingly, when we disrupt ribosome and ribosomal RNA production, processes that have a direct influence on the nucleolus, we noted analogous chromatin accessibility changees in the non-reproductive (somatic) cells of the organism. These alterations closely parallel the deposition of H3K4 methyl marks observed in response to UV radiation.
Question
What's the connection between disrupting ribosome production and the occurrence of H3K4 methylation, a phenomenon linked to both aging and the DNA damage response triggered by UV radiation?
Additionally, we have generated the first complete deletion of the ribosomal DNA locus in a metazoan, C. elegans, is the seeder of nucleolus structure. This is valuable for investigating epigenetic function of nucleolus in a multi-cellular organism. Ribosomal DNA locus deleted embryos complete embryogenesis based on maternal ribosomes yet they lack a distinct nucleoli structure, thus enabling a valuable tool to investigate nucleolar function in a multi-cellular organism.
Our Mission
The Sarinay Cenik Lab is a dynamic, diverse and innovative team of researchers.We foster a respectful, friendly and fun research environment where everyone can thrive. Our goal is to conduct high-quality, reproducible science that will have a lasting impact on the field. We believe that training the next generation of scientists is essential, and we're dedicated to providing them with exciting research plans.
Joining our lab
We are always looking for highly motivated junior and experienced scientists who are interested in fundamental questions in cell and developmental biology with backgrounds in genetics, computational biology, cell biology, or biochemistry. If interested, please email your CV along with your references to esarinayATutexas.edu